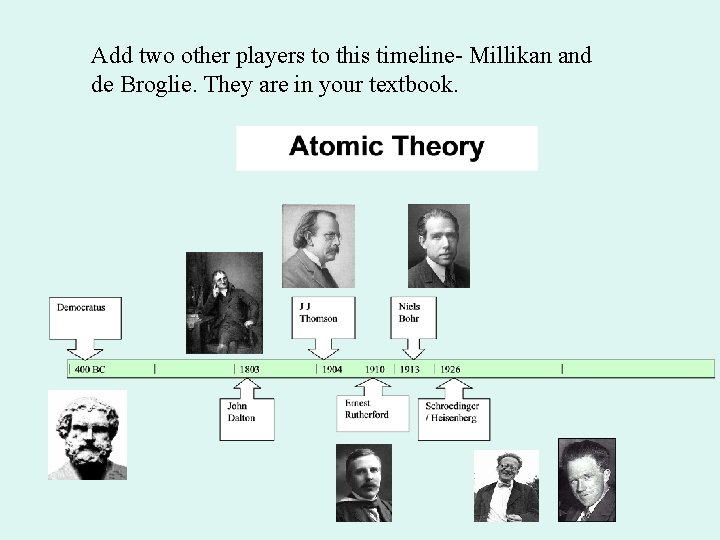
Answer Key For Atomic Timeline Events In
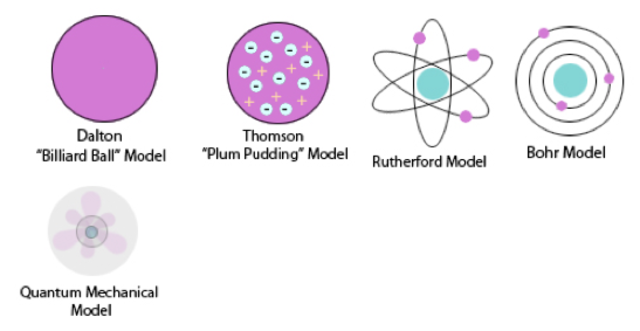
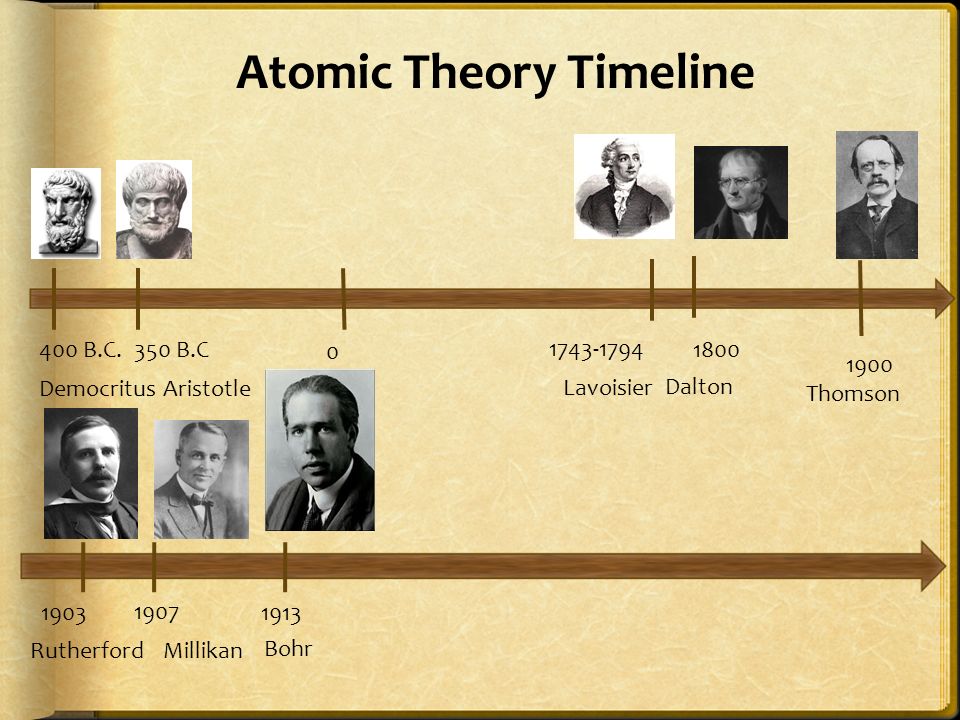
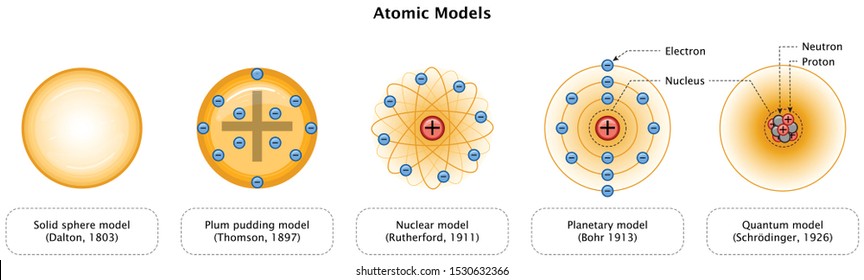
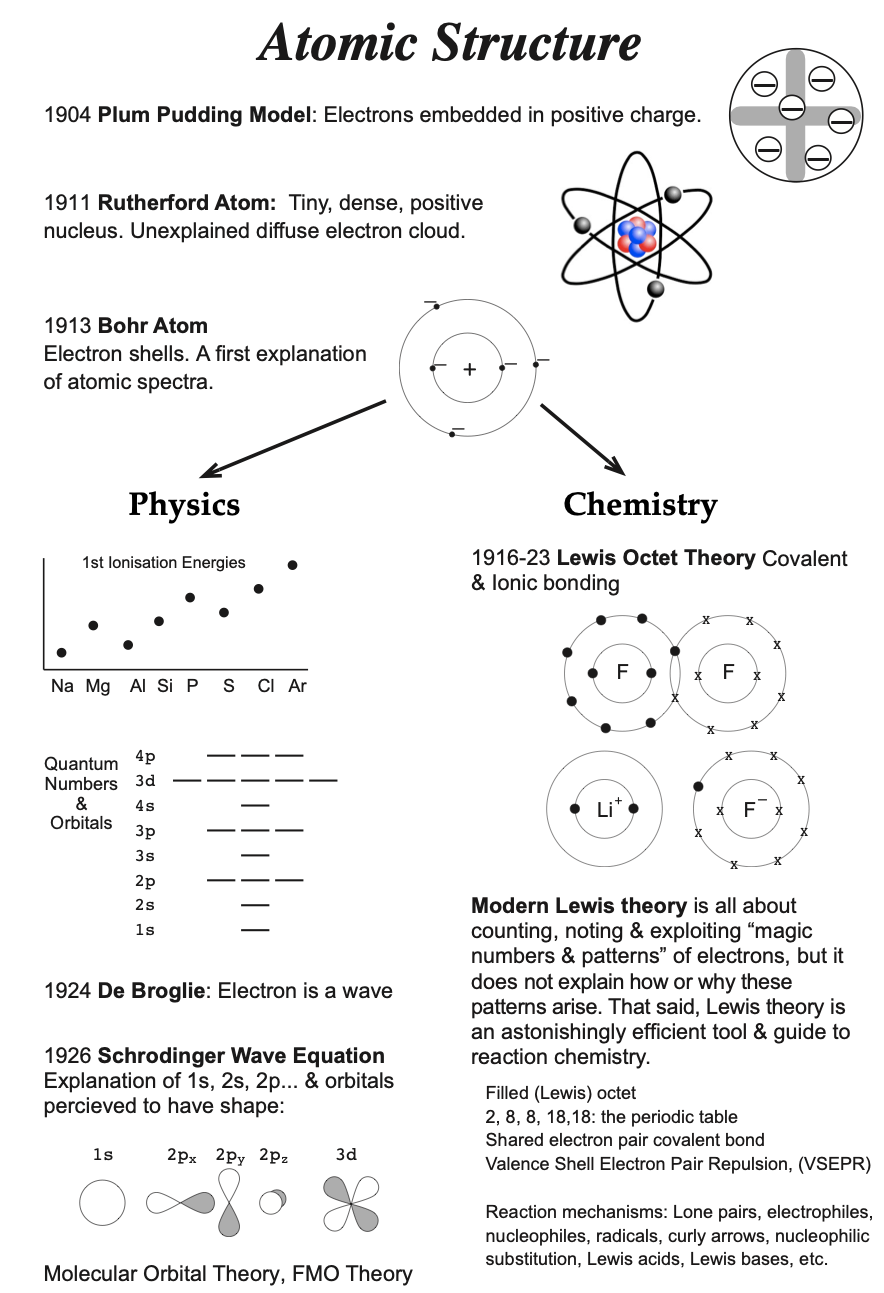
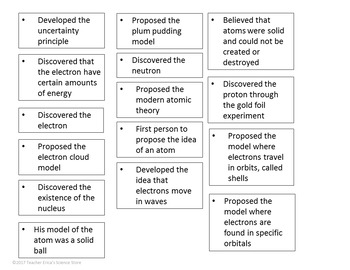
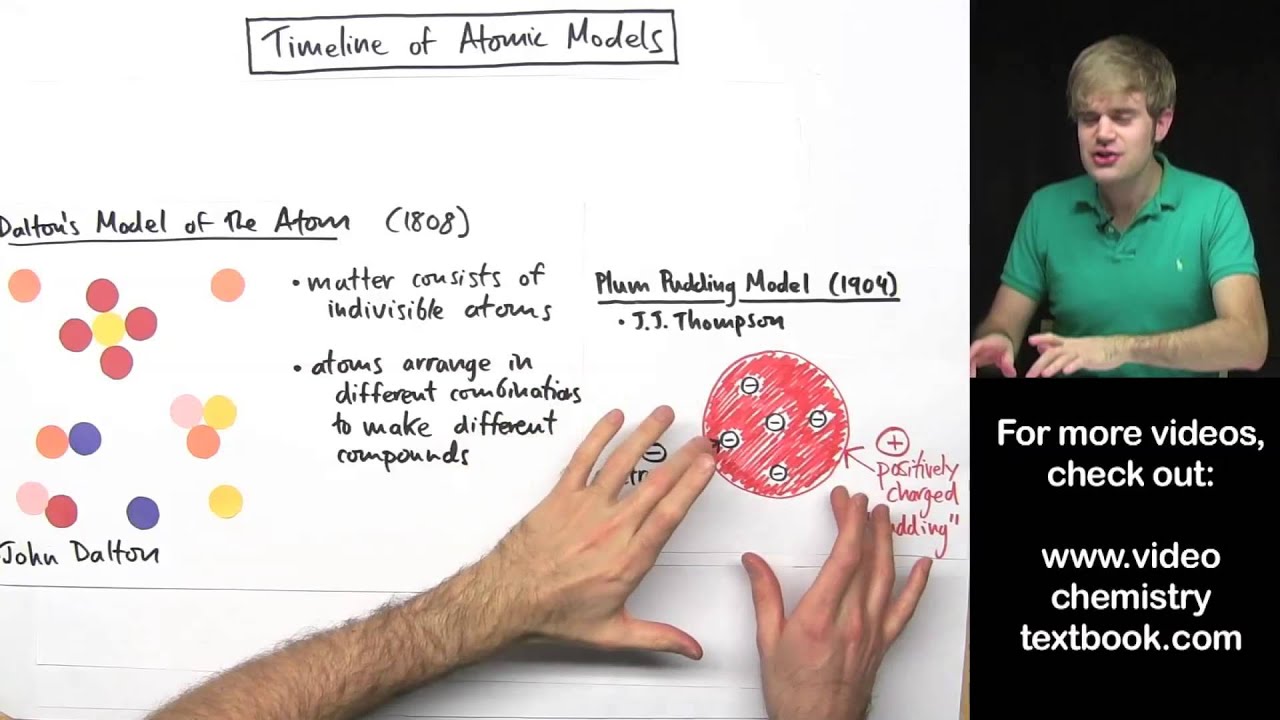
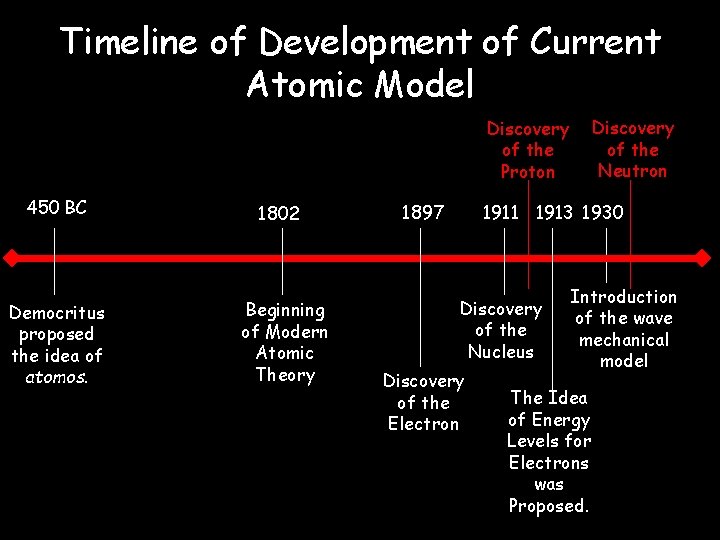
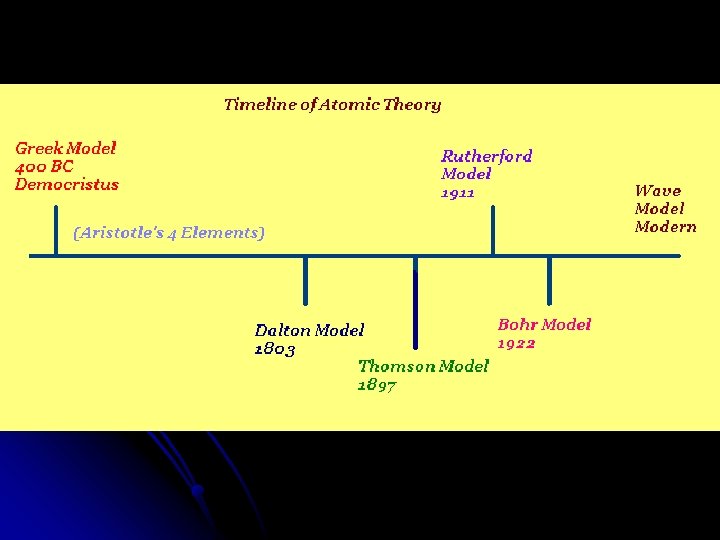
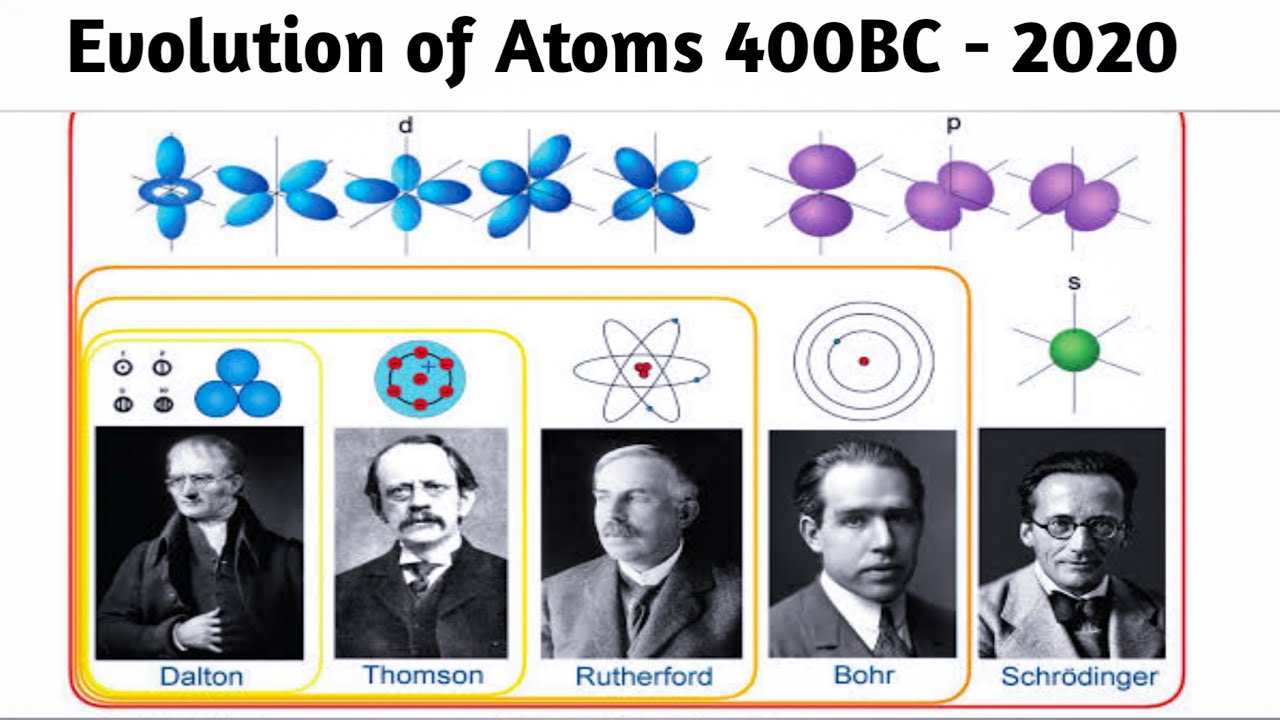
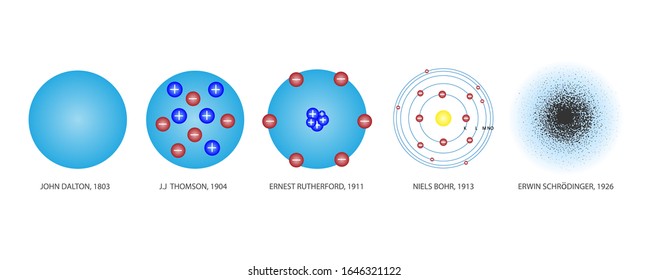
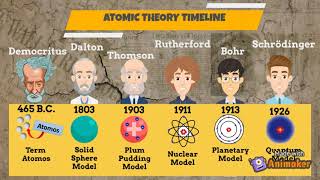
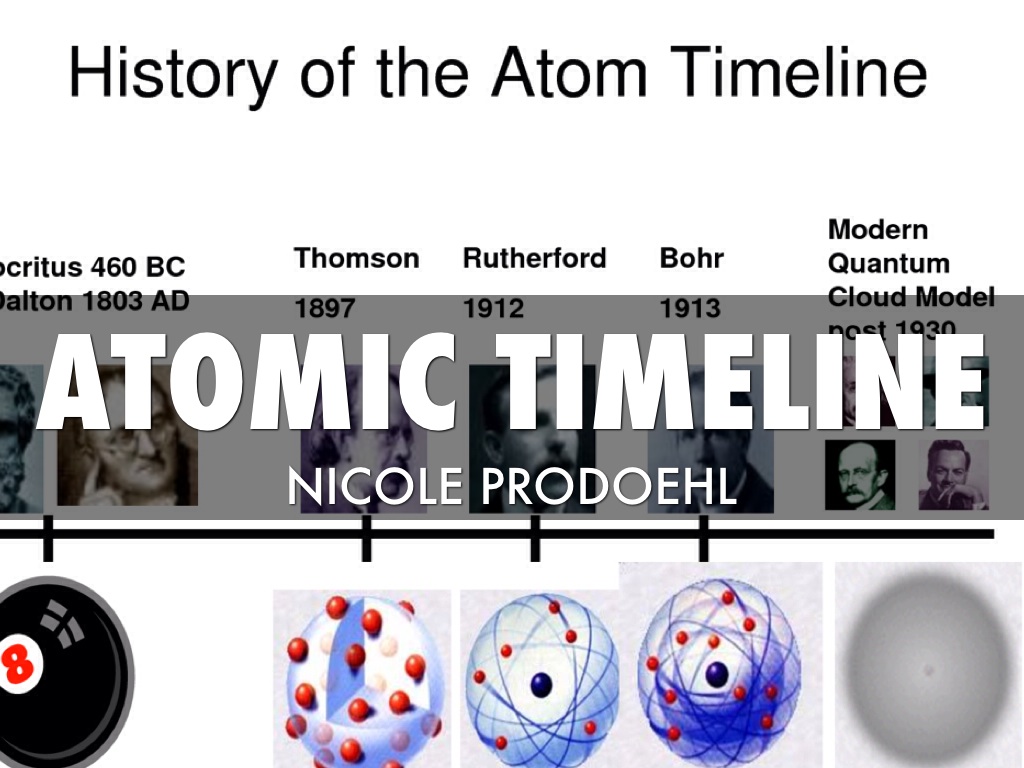
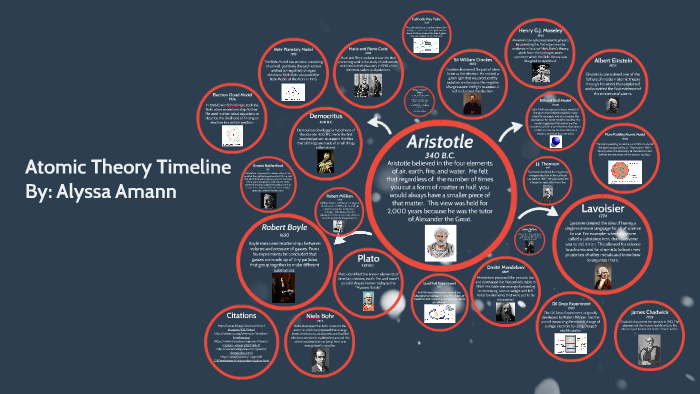
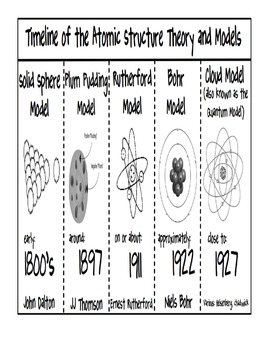
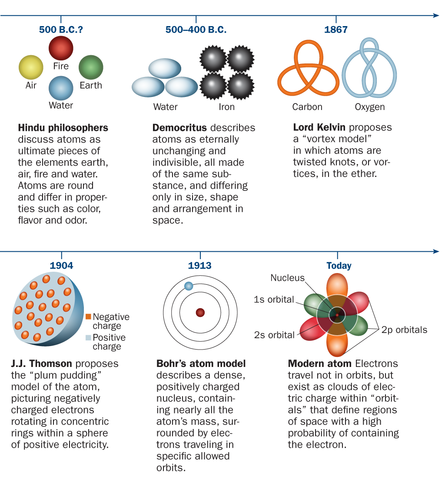
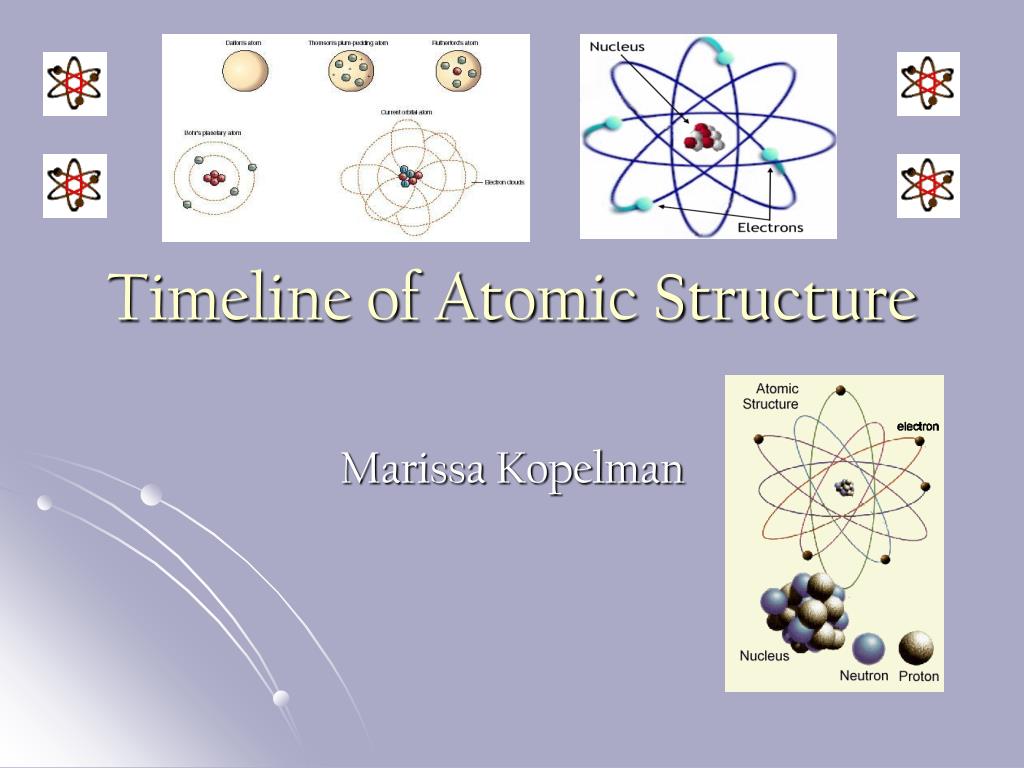
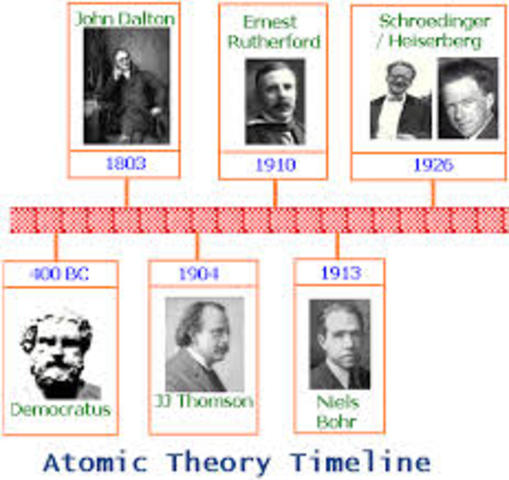
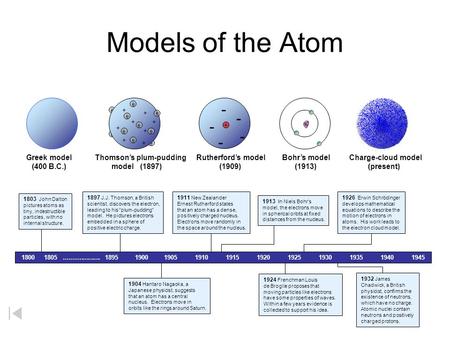
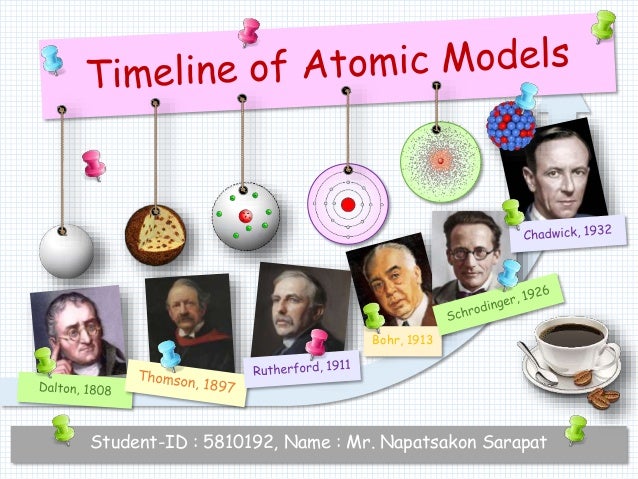
Model, the atom was like a plum pudding it was mostly a thick, positively charged material, with negative electrons scattered about it like plumsin a pudding In 908, Ernest Rutherford took an extremely thin sheet of gold foil and bombarded it with electrons Much to his surprise, most of the electrons went right through the foil, and the occasional bullet or electron was seriouslyAtomic Theory Timeline The atomic model has changed over time For over two centuries, scientists have created different models of the atom As scientists have learned more and more about atoms, the atomic model has changed Atomic Theory Timeline Here is a timeline of some of the major ideas Dalton Thomson Rutherford Bohr Chadwick Modern But First, Democritus!
Model of atom timeline
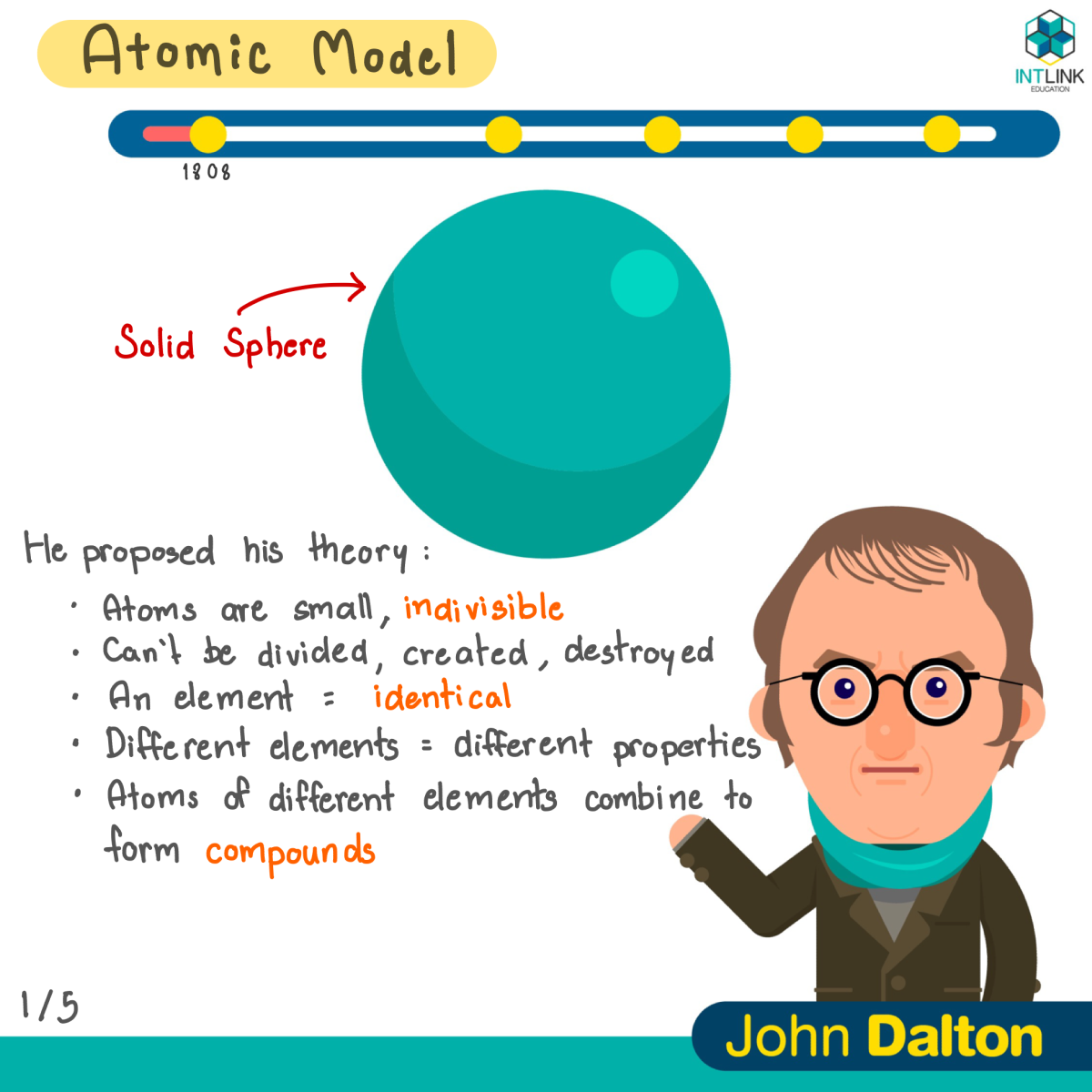
Model of atom timeline-Atoms of the same element are identical in terms of mass and chemicalAtomic theory timeline Activity for students to learn (or revise) the evolution of the atom Focuses on discoveries required for AQA additional physics (P251 atomic structure), including plum pudding model, Rutherford's gold foil experiment, and the subsequent Rutherford and Bohr models The first two pages of the word document is a summary

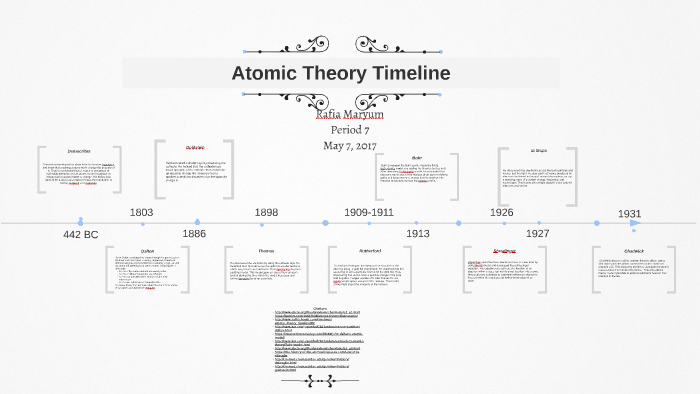
Atomic Theory Timeline By Emilie Jacobus
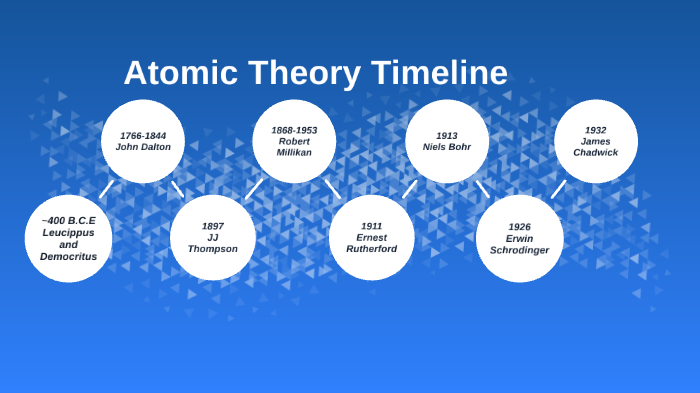
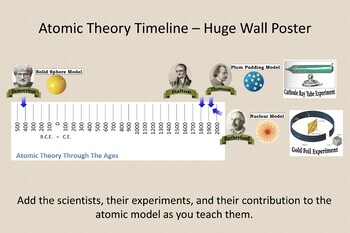
Students will analyze models and describe the motion of particles in solids, liquids, and/or gasses Agenda Bell Ringer Atoms PowerPoint Interactive Notebook Notes ThinkPairShare Brain Pop Atomic Theory Gallery Walk Activity Independent Practice Atoms Matter is anything that takes up space and has mass All matter is made of atoms Atoms are the basic building blocks of matterModels of the Atom Timeline – This video is about the different ways that scientists have pictured the atoms over the years It starts with Democritus and Leucippus, the first philosophers to discuss atoms The video also covers the work of Dalton, Thompson, Rutherford, Niels Bohr, and Schrodinger The Law of Conservation of Mass The law of conservation of mass states that massAtomic Model Timeline Print PDF Zoom Out Main Albert Einstein 1905 •plum pudding model the atom was positive and there were negative forces wandering around itdiscovered atoms of the same element but different atomic weight Planck 1900 •Quantum Theory •energy is given off in little packets •energy in wave form is restricted to specific quantities •led to understanding of
19 Werner Heisenberg (1927) Quantum Model of the Atom Timeline Albert Einstein (1905) discovered it is impossible to determine the exact location and velocity of an electron at the same time uncertainty principle creator of quantum mechanics noble prize 1932 this meme nowModels of the atom AQA The idea of the atom as the building block of matter has developed over time What was thought of as a single particle about 1 ×The Plum Pudding Model • Thomson did not know how the electrons in an atom were arranged He believed they were mixed throughout an atom • He proposed that the atom was a sphere of positively charged material Spread throughout the atom were the negatively charged electrons similar to plums in a pudding or chocolate chips in ice cream
Model of atom timelineのギャラリー
各画像をクリックすると、ダウンロードまたは拡大表示できます
 |  |  |
 |  | |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
:max_bytes(150000):strip_icc()/GettyImages-1157225833-01064c770b904b23bb07df6eec68f35d.jpg) | 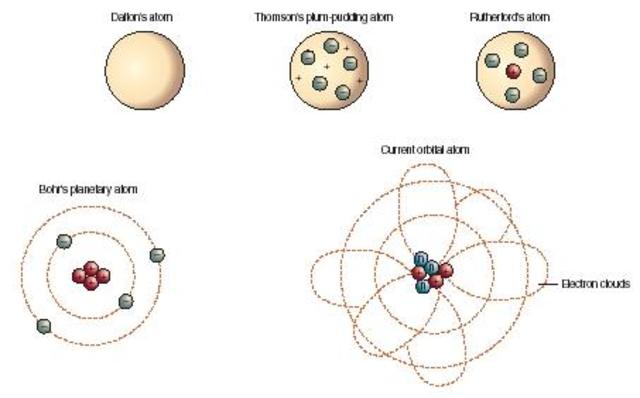 | |
 |  | |
 |  | |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  | |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  |  |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 | ||
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |  |  |
 |  |  |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  |  |
 |  | |
 |  | |
「Model of atom timeline」の画像ギャラリー、詳細は各画像をクリックしてください。
 |  | |
 |
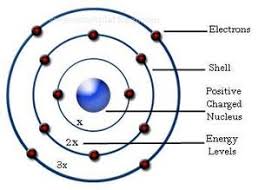
Bohr, Danish physicist, came up with his own model of the atom based off of Rutherford's model, the Bohr model This model adapts Rutherford's nuclear structure to Max Plank's Quantum Theory Through his model introduced the idea of energy levels in the atom and that an electron could drop from a higherenergy orbit to a lower one, in the process emitting a quantum of discrete energyQ This was the first model of the atom ever proposed It was simple and described atoms as tiny spheres that could not be broken down into smaller pieces answer choices Democritus's model of the atom The Plum Pudding Model of the atom The Rutherford Model of the atom The Quantum Mechanical Model of the atom
Incoming Term: model of an atom timeline, atom model history timeline, atom model timeline, model of atom timeline, model of the atom timeline,




0 件のコメント:
コメントを投稿